FDA Announces Nutrition Facts Label Compliance Date
What the FDA's new label compliance dates mean for your labeling.Late in 2018, the FDA announced that Jan. 1, 2022 will be the uniform compliance date for final food labeling regulations that are issued in calendar years 2019 and 2020.
VIA the FDA’s December 2018 Constituent Update:
The U.S. Food and Drug Administration (FDA) announced today that January 1, 2022, will be the uniform compliance date for final food labeling regulations that are issued in calendar years 2019 and 2020. All food products subject to the January 1, 2022, uniform compliance date must comply with the appropriate labeling regulations when initially introduced into interstate commerce on or after January 1, 2022. This action does not change existing requirements for compliance dates contained in final rules published before January 1, 2019.
As you are likely aware, the FDA occasionally issues regulations that require changes to the way manufacturers label food. Along with these updates, the FDA has also issued revisions to regulations in order to “correct errors made in some sample label illustrations, restore several inadvertent deletions, correct citations to three cross-references, and remove a sentence regarding the font size and bolding requirement for the “Calories” declaration in dietary supplement labels.”
What the FDA’s Nutrition Facts Label Compliance Dates Mean for Your Labeling.
In short, if you are in food manufacturing, you should know what these changes entail. Nutrition facts label compliance can seem daunting at times, but is a vital element to any food manufacturer’s supply chain. You should also plan accordingly so your operations are prepared when these compliance mandates come into effect.
These new issuances also include rules and minor revisions to previous regulations:
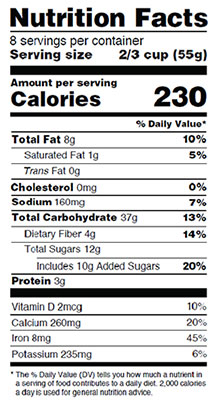
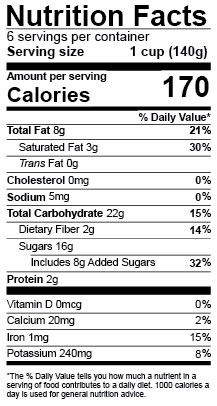
For example, some sample Nutrition Facts labels included a line directly beneath “Saturated Fat” that did not extend completely to the left edge of the label, and one sample label omitted information regarding the number of servings per container as well as the serving size. A sample Supplement Facts label listed “sucrose,” rather than “sugar,” in the ingredients list.
For a lot of manufacturers, including those in the food industry, it can be a real struggle to maintain different label files and formats. As labels become more diverse and complex, it’s important to keep your labeling procedures organized. That can be difficult when the number of compliance labels you have in your queue is growing by the minute. Finding simple solutions to automate label printing is vital for food manufacturers and distributors in order to adhere to FDA nutrition facts label compliance mandates.
That’s where solutions like MarkMagic’s PrintTransformer add-on comes in handy. With PrintTransformer users can dynamically change fields based on label or document information. Contact us to request a free trial of PrintTransformer today.