Healthcare is one of the most complex and highly regulated industries—and medical device labeling plays a critical role in patient safety and compliance. FDA recall analyses show that roughly 8–10% of medical device recalls are linked to labeling-related issues, such as mislabeling, missing information, or incorrect instructions.
These errors can lead to device misuse, delayed care, and increased risk to patients, underscoring why labeling accuracy is far more than a regulatory checkbox.
Medical device labeling encompasses a wide range of essential information, including device identification, usage instructions, warnings, and regulatory and traceability data. When any of this information is inaccurate or unclear, the consequences can extend across healthcare teams, supply chains, and patient outcomes.
This starter guide provides an in-depth look at the key elements, regulations, and best practices for medical device labeling, serving as a practical resource for manufacturers, regulatory and quality teams, and anyone involved in bringing compliant, safe medical devices to market.
What is Medical Device Labeling?
Medical device labeling refers to the written, printed, or graphic information that accompanies a medical device and communicates essential details about its identity, use, safety, and regulatory status. This includes the label affixed directly to the device, packaging labels, instructions for use (IFU), manuals, and any digital or electronic documentation provided to users.
Labeling plays a critical role in ensuring that healthcare professionals, patients, and caregivers can safely and effectively use the device. It includes information such as:
- Device name and model
- Manufacturer details
- Unique Device Identification (UDI) code
- Intended use and indications
- Warnings and precautions
- Usage instructions and diagrams
- Expiration dates and sterilization methods
- Regulatory symbols and certifications (e.g., CE mark, FDA clearance)
Because medical devices are often used in high-stakes clinical environments, accurate, accessible, and compliant labeling is essential—not only for patient safety but also for meeting the strict requirements of regulatory bodies like the FDA, EU MDR, and others.
Key Statistics on Medical Device Labeling
Labeling issues are a significant concern in the medical device industry, accounting for roughly 8% of all FDA recalls and contributing to approximately 33% of medication errors, with around 30% of related fatalities linked to these mistakes.
Regulatory Standards
Medical device labeling is governed by a complex web of international regulations and standards designed to ensure safety, transparency, and traceability. These rules vary by region but share the common goal of protecting patients and informing users.
Below are the key regulatory standards and frameworks that manufacturers must comply with when labeling medical devices:
FDA (U.S. Food and Drug Administration) – 21 CFR Part 801 & UDI Rule
- 21 CFR Part 801 outlines labeling requirements for medical devices marketed in the U.S., including proper identification, instructions for use, and warnings.
- The Unique Device Identification (UDI) Rule mandates that most medical devices carry a UDI code for improved traceability and post-market surveillance.
- 21 CFR Part 11 applies if electronic records or electronic signatures are used in labeling workflows.
European Union Medical Device Regulation (EU MDR 2017/745)
- The EU MDR imposes strict requirements for labeling medical devices sold in Europe.
- Labels must include:
- UDI-DI and UDI-PI information
- Manufacturer and importer details
- Symbols conforming to EN ISO 15223-1 and ISO 20417
- Language localization for each EU country where the device is sold
- Instructions for use (IFU) must be clear and accessible, with electronic IFUs allowed under specific conditions.
ISO Standards
Several ISO standards define how medical device labels should be structured and what information must be included:
- ISO 15223-1: Defines standardized symbols used on labeling to ensure international understanding and reduce language dependency.
- ISO 20417: Specifies the information that must be supplied by the manufacturer, including labeling and IFU content.
- ISO 13485: Focuses on quality management systems for medical devices, including labeling as part of production and post-production controls.
Other Regional Requirements
- Health Canada: Requires bilingual (English/French) labeling and adherence to Canadian Medical Devices Regulations (CMDR), including UDI under upcoming adoption.
- TGA (Australia): Enforces labeling standards aligned with the Global Harmonization Task Force (GHTF) and mandates UDI for certain risk classes.
- China NMPA and Japan PMDA: Have their own country-specific rules regarding UDI, traceability, and required documentation.

The Importance of Medical Device Labeling
Medical device labeling goes beyond regulatory checkboxes—it actively safeguards proper, safe, and compliant device use across global markets. Clear and effective labels help healthcare providers communicate accurately, protect patients, and maintain traceability throughout a device’s lifecycle.
Below are some of the key reasons why proper labeling is essential in healthcare environments:
- Patient Safety: Accurate labeling is fundamental for patient safety. It provides crucial information about the correct use of the device, potential risks, and any necessary precautions. Clear and concise instructions contribute to preventing misuse or errors that could jeopardize patient well-being.
- Regulatory Compliance: Regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have stringent requirements for medical device labeling. Compliance with these regulations is not only mandatory but also essential for gaining market approval and maintaining product legality.
- Communication in Healthcare Settings: Medical device labels facilitate effective communication among healthcare professionals. Whether in a hospital setting or a clinical environment, clear labeling helps medical staff quickly and accurately identify devices, understand their intended use, and follow proper procedures.
- Product Traceability: Accurate labeling aids in product traceability throughout the supply chain. From manufacturing to distribution and usage, each labeled device can be tracked, ensuring quality control and regulatory adherence at every stage.
Free Barcode Generator →
Generate scannable barcodes in seconds. Download the barcode image and import it directly into MarkMagic to use across labels, electronic forms, and reports.
Key Elements of Medical Device Labeling
Medical device labeling forms the foundation for patient safety, regulatory compliance, and clear communication across the healthcare ecosystem. At a high level, effective medical device labels must include:
- Clear device identification and traceability details
- Defined intended use
- Easy-to-understand instructions for use
- Appropriate warnings and precautions
- Manufacturer contact information
- Required regulatory and compliance markings
Below is a closer look at each key element.
Device Identification
Every medical device label must include clear and unambiguous information about the device’s identity. This typically involves the device name, model number, and any relevant serial or lot numbers. This information is crucial for traceability and differentiation from other devices.
Intended Use
The label should explicitly state the intended use of the medical device. This information guides healthcare professionals and end-users in understanding the device’s purpose and ensures that it is utilized appropriately.
Instructions for Use
Clear and concise instructions for use are imperative for patient safety. This section details the proper procedures for device operation, preparation, and any necessary precautions or warnings. The language should be easily understandable by the intended audience, which may include patients, caregivers, or healthcare professionals.
Warnings and Precautions
Medical devices often come with inherent risks. Warnings and precautions on the label communicate these risks to users, providing information on how to mitigate them. This section is critical for preventing adverse events and ensuring safe device usage.
Manufacturer Information
The label should prominently display information about the device manufacturer, including the company name, address, and contact details. This information is crucial for regulatory purposes and allows users to reach out for support or additional information.
Regulatory Information
Device labeling must include regulatory information, such as the device’s regulatory status (e.g., FDA clearance or CE marking), applicable standards, and any symbols indicating conformity with specific regulations.

ISO 15223-1 Symbols
Symbols and Icons
The use of standardized symbols and icons on medical device labels enhances international understanding. Symbols can convey information about actions, warnings, and other instructions without relying on language, improving accessibility for a global audience.
What is a UDI Label?
A UDI (Unique Device Identification) label is a distinctive code assigned to medical devices to provide a standardized and globally recognized identifier for each specific product. Regulatory bodies established the UDI system to enhance traceability and overall safety of medical devices.
The UDI consists of a combination of numeric or alphanumeric characters that uniquely identify a device, along with additional information such as the device’s expiration date and batch or lot number. The label is designed to be easily readable and electronically accessible, facilitating quick and accurate identification of medical devices by healthcare providers, regulators, manufacturers, and other stakeholders.
Implementation of UDI labels is mandated by regulatory bodies in various regions, such as the U.S. Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in the European Union, to enhance post-market surveillance, streamline recalls, and improve patient safety.
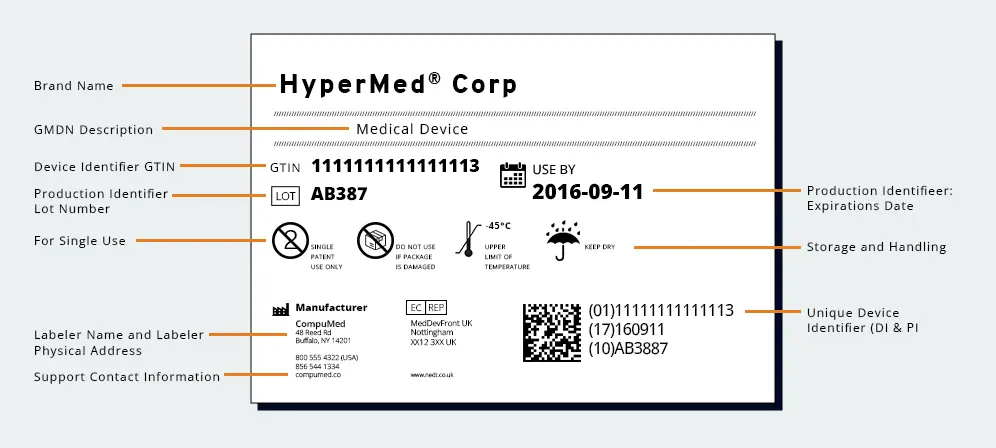
UDI Label
Best Practices in Medical Device Labeling
Proper labeling is essential for ensuring accuracy, compliance, and effective communication in the healthcare industry. Here are some best practices for medical device labeling:
User-Centered Design
Design labels with the end-user in mind. Use clear and concise language, employ legible fonts, and consider the visual hierarchy to emphasize critical information. Conduct usability testing to ensure that labels are easily understood by the intended audience.
Comprehensive Risk Analysis
Conduct a thorough risk analysis to identify potential hazards associated with the device. This analysis should inform the inclusion of warnings, precautions, and other risk-mitigation measures on the label.
Periodic Label Reviews
Medical device labels should undergo regular reviews, especially when there are updates to the device or changes in regulations. This ensures that the information remains accurate, up-to-date, and compliant with current standards.
Localization Considerations
For devices intended for international markets, consider localization factors such as language translations, cultural sensitivities, and compliance with regional regulations. This ensures that the label remains effective and compliant in diverse markets.
Collaboration with Regulatory Experts
Work closely with regulatory affairs professionals who are well-versed in medical device regulations. Collaboration with regulatory experts helps ensure that the labeling process aligns with regulatory requirements and facilitates a smoother path to market approval.
Streamlining Your Medical Device Labeling
Organizations in the medical device industry can streamline and optimize their labeling processes by leveraging MarkMagic, a robust and versatile labeling software solution. MarkMagic offers a comprehensive platform that empowers businesses to efficiently design, manage, and print labels for medical devices.
With its user-friendly interface and a wide range of design tools, organizations can create compliant and visually effective labels that meet regulatory requirements.

MarkMagic supports dynamic data integration, allowing seamless connectivity with databases and systems to ensure accurate and up-to-date information on labels. Furthermore, the software facilitates compliance with global standards, such as UDI (Unique Device Identification) requirements, ensuring that organizations meet regulatory obligations.
MarkMagic’s versatility extends to label printing across various devices and printers, providing flexibility in production environments.
By incorporating MarkMagic into their operations, organizations can enhance labeling accuracy, improve regulatory compliance, and streamline the overall efficiency of their medical device labeling processes.
Better Labeling, Faster Printing.
Ensure every label is accurate, compliant, and printed without delays. Discover how CYBRA’s barcode and printing solutions streamline operations and eliminate errors.
















