
In the healthcare industry, ensuring the safety, efficacy, and traceability of medical devices is paramount. One of the pivotal frameworks facilitating this is the Unique Device Identification (UDI) system. Established by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), UDI compliance is more than a regulatory requirement—it is a cornerstone of modern healthcare. In this article, we’ll go over why UDI compliance is important and how manufacturers can stay compliant with governmental and industrial regulations.
UDI compliance is crucial in the medical device industry as it enhances patient safety, streamlines supply chain management, and supports regulatory oversight. By providing a standardized identification system, UDI ensures accurate tracking and traceability of medical devices, enabling swift action in case of recalls or adverse events.
Specific UDI Compliance Benefits
- Enhanced Patient Safety
- Improved Traceability
- Streamlined Supply Chain Management
- Regulatory Oversight Support
- Global Trade Facilitation
This system improves inventory management, aids in post-market surveillance, and aligns with international standards, facilitating global trade and ensuring high-quality care. Ultimately, UDI compliance fosters a safer healthcare environment by ensuring that medical devices are reliably identified and monitored throughout their lifecycle.
What is Unique Device Identification (UDI)
The Unique Device Identification (UDI) system is a globally standardized framework designed to ensure the traceability, safety, and efficiency of medical devices throughout their lifecycle. Established by regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), UDI serves as a critical tool in the healthcare sector. Here’s an in-depth look at what UDI entails and its significance.
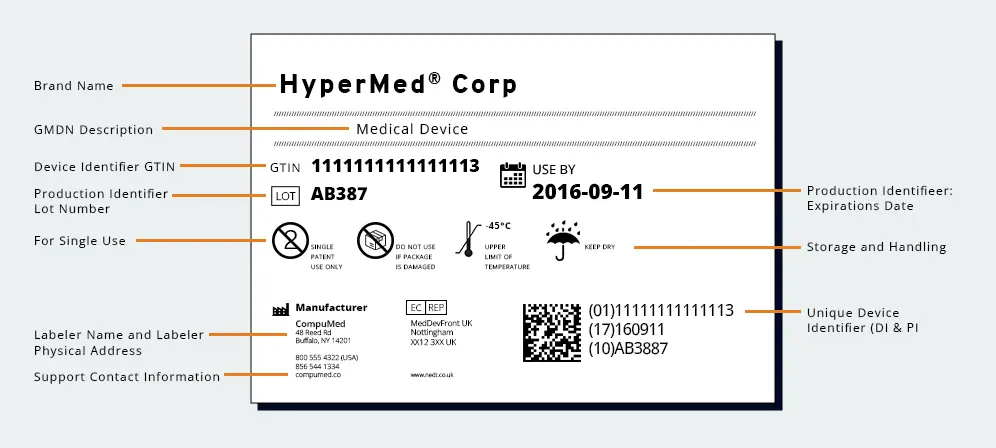
Enhancing Patient Safety
The primary objective of UDI is to enhance patient safety. By providing a standardized identification system, UDI enables accurate tracking and identification of medical devices throughout their lifecycle. In the event of a recall, healthcare providers can swiftly identify affected devices, ensuring that corrective actions are taken promptly and efficiently. This traceability mitigates risks associated with faulty or harmful devices remaining in circulation, thereby protecting patients from potential harm.
Streamlining Supply Chain Management
UDI compliance significantly improves supply chain management. With a unique identifier for each device, manufacturers, distributors, and healthcare providers can maintain precise inventory records. This level of detail aids in preventing stockouts and overstock situations, optimizing the availability of essential medical devices. Furthermore, UDI facilitates better forecasting and planning, leading to more efficient operations and reduced costs.
Supporting Regulatory Oversight
Regulatory agencies rely on UDI to monitor the performance and safety of medical devices. The system provides a comprehensive database where adverse events, recalls, and safety alerts can be linked to specific devices. This data-driven approach enables regulators to identify trends and potential issues more effectively, leading to quicker regulatory actions and the continuous improvement of device standards.
Enhancing Post-Market Surveillance
Post-market surveillance is crucial for assessing the long-term performance of medical devices. UDI aids in this by ensuring that all devices in the market are easily identifiable. This identification is essential for tracking the real-world performance of devices, collecting post-market data, and conducting epidemiological studies. The insights gained from these activities help in refining device designs and improving future iterations, ultimately benefiting patient outcomes.
Facilitating Global Trade
In an increasingly globalized market, UDI compliance aligns with international standards, making it easier for manufacturers to enter new markets. Harmonization of UDI requirements across different jurisdictions simplifies the regulatory submission process and ensures that devices meet global safety and quality standards. This international alignment not only boosts trade but also ensures that patients worldwide have access to high-quality medical devices.

Empowering Healthcare Providers
Healthcare providers benefit significantly from UDI compliance. With access to detailed information about each device, including its production history, expiration date, and specific characteristics, providers can make more informed decisions regarding patient care. Additionally, in cases where devices need to be replaced or upgraded, the UDI system ensures that compatible and safe alternatives are readily identifiable.
Improving Clinical Outcomes
The integration of UDI into electronic health records (EHRs) and other health IT systems enhances the accuracy of patient records. This accuracy is vital for effective clinical decision-making and personalized treatment plans. By ensuring that the correct devices are used for each patient, UDI contributes to improved clinical outcomes and a higher standard of care.
Conclusion
UDI compliance is a transformative element in the medical device industry, driving advancements in patient safety, regulatory oversight, and operational efficiency. By ensuring the traceability and accountability of medical devices, UDI fosters a safer and more reliable healthcare environment. As the industry continues to evolve, adherence to UDI standards will remain a critical component in delivering high-quality care and enhancing patient trust in medical technologies.
Better Labeling, Faster Printing.
Ensure every label is accurate, compliant, and printed without delays. Discover how CYBRA’s barcode and printing solutions streamline operations and eliminate errors.










 RFID Cage
RFID Cage